New insights on the dynamic skin microbiome and commensalism, rather than static colonizers, have been prevailing scientific research and precedent notion of bacterial colonization as a primary etiology of acne vulgaris outlives its pragmatism. Skin surfaces in the acne prone areas, face, shoulders, upper chest and back, are harbored by Staphylococcus epidermidis, S. epidermidis, and anaerobic gram positive Cutibacterium acnes, C. acnes, previously known as Propionibacterium acnes.
On the other hand skin colonization with staphyloccocus aureus, by some studies, have been implicated in pathogenesis of acne vulgaris. In adolescence, the number of bacteria on the skin surface increases while sebaceous areas are primarily resided by C. acnes whose elimination targeted by most acne conventional therapeutics for acne.
Next generation sequencing of acne bacteria, NGS, has helped distinguish six strains of C. acnes, IA1, IA2, IB, IC, II and III, with distinct microbiology and host interacting qualities. Genetically analyzed, certain strains were suggested possessing more virulence factors and perceived as pathological than others, found to be associated with healthy states. Studies suggest that bacteria have nothing to do with the initiation of comedogenesis. However, acne bacteria, in particular, may in some situations be important in the initiation of inflammation especially in cysts.
A commensal, pleomorphic and anearobic rod incapable of tissue invasion and noteworthy infection, C.acnes, is, in large, a component of normal skin flora. Metagenomic research indicates that C.acnes is the predominant bacteria of pilosebaceous glands with the capability to catabolize triglycerides and harvesting glycerol yet lack of enzymes to utilize fatty acids as a carbon source is evident.
Staphylococcus epidermidis leverges its catabolites to dwindle C.acnes colonies to wallow in its superiority as the predominant commensal of the human skin. By metabloizing glycerol, S.epidermidis develops inhibtion zones threatening to survival of C.acnes, suggestive of how these two microorganisms competitive coexistence is warranted for a healthy skin.
Over the past decade, role of C. acnes in acne vulgaris has been largely debated and certain novel insights outpower precedent thinking with the skin microbiome dysbiosis gaining more traction. Proof for C. acnes colonization in acne vulgaris found itself in a mire of equivocality while elimination of the bacteria resulted in utter alleviation of acne lesions. Loss of diversity in strains of C. acnes takes a more favorable stance and appears more promising but still seeks more substantiation.
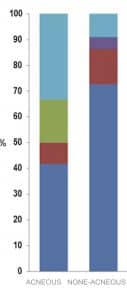
Lack of bacterial diversity in acne lesions
C. acnes is equipped with several lipolytic enzymes which can alter epidermal milieu, not always harmlessly. Two lipases has been identified, triacylglycerol lipase GehA and GehB, capable of hydrolysing triglycerides into free fatty acids which may contribute to inflammation and comedogenesis. However, sebum free fatty acids have been found to be protective by enhancing expression of human beta denfensin, hBD-2 and hBD-4, in sebocytes. Moreover, several glycolipid processing enzymes, putative endoglycoceramidases, identified in C. acnes, are accounted for catalyzing ceramides and glycosphingolipids for provision of carbohydrate as energy source for the bacteria.
Three main phylotypes of C. acnes have been identified, I, II and III and type I is subdivided into IA1, IA2, IB and IC. Type IA and some subgroups of IB has been found in most moderate to severe acne lesions while type II and III are mostly associated with healthy skin. Lipase of IA strains was reported architecturally truncated and distinguished in virulence and quantitative activity while lipase amid other strains was suggested structurally conserved. Increased lipase activity leads to further generation of free fatty acids from triglycerides and lysophosphatidyl choline, advancing to inflammatory processes. These findings further open up the tantalizing contingency of plastic cutaneous microflora that one strain outpopulates another when sebaceous or inflammatory markers are lured to transformation.
Characteristically, C. acnes appears to form biofilms, generated by a network of polysacharide to adhere to follicular keratinocytes, which confirms them not just colonization and comedogenicity but contumacy to antimicrobial agents. This glycocalyx network substantiates in that it can alter the course of acne disease and its therapeutic approaches by inducing keratinocytes adhesiveness to give rise to comedones and by upregulation of beta-1 ntegrins and impacting keratinocytes differentiation.
Furthermore, S. epidermidis invokes TLR2 to increase AMPs, hBD2 and hBD3, concurrent with consolidation of tight junctions barrier of epidermal keratinocyte to relent cutaneous vulnerability to various pathogens. Tight junctions turn less permeable upon facing multitude of proinflammatory cytokines such as IL-1, IL-12, TNF-α, IFN-γ while anti inflammatory TGF-β and IL-10 heighten the epidermal influx which suggests how dynamic control of tight junctions is.
Among other metabolites of C. acnes are short-chain fatty acids, SCFAs, propionate, acetate, butyrate and valerate with salutary as well as deleterious impacts on the host. SCFAs can limit growth of staphylococcus aureus on the skin by acidification of the skin and compromising an ideal abode for growth of S. aureus, however, SCFAs perniciousness is not limited to the skin pathogens, and it risks health of epidermal barrier function.
Similar findings indicate that SCFAs can induce cytokine expression and an exaggerated cytokine response to toll-like receptor 2, TLR-2, activation in keratinocytes by suppression of histone deacetylase activity concurrent with stimulation of fatty acid receptors. In jarring discord to keratinocytes, sebocytes free fatty acid receptors under anaerobic condition of pilosebaceous units may be evoked by C. acnes production of free fatty acids which, in turn, trigger inflammatory processes.
Toll like receptors, TLR-2, and matrix metaloproteinases have been identified as targets of acne bacteria to breed inflammation and incite cytokines. Activation of TLR-2 on keratinocytes by C. acnes biofilms followed by induction of IL-6, IL-8 and TNF-α has been reported to be under regulation by a microRNA, miR-146a.
Nonetheless, acne bacteria may resort to other means to embody its pathogenecity as in perifollicular dermis induces monocytes to release IL-1β by activation of NLRp3 inflammasomes, a process which is potassium-ion efflux and caspase-1 dependent. While IL-1α was found in open comedones and instigate follicular hyperkeratinization, IL-1β can provoke sebocytes to deliver proinflammatory cytokines, IL-6 and IL-8 and further foment inflammation.
S. epidermidis, a skin commensal, pays tribute to cutaneous anti-inflammatory processes by challenging C. acnes procytokinetic menaces, induced by provocation of keratinocytes to release IL-6 and TNF-α via inhibition of TLR2. Another mechanism S. epidermidis is resorted to appears to be glycerol fermentation and generation of intermediates such as succinic acid to compromise amenities of its cohabitation with C. acnes.
Moreover, it was shown that intralesional or topical application of succinic acid limit growth of acne bacteria. Probiotics with S. epidermidis may be therapeutically employed against C. acnes in acne vulgaris as suggested by some studies which corroborate role of short-chain fatty acids, bred by the bacteria, to confine pilosebaceous inflammation.
Saccharomyces Cerevisiae, S. cerevisiae, has substantiated a role in pathogenesis of number of cutaneous diseases and its significance turns into a fulcrum for numerous dermatological studies based on its immunomodulatory characteristics. Amid several, a link between this eucaryotic cell, psoriasis and its treatment have been found, denoting decline or absence of S. cerervisiae in psoriatic patients. In addition, antibodies against gp200, a structural glycoprotein in S. cerevisiae, has been delineated in patients with atopic dermatitis. While another study proposes an association between consumption of S. cerevisiae fermented product and de-escalation of inflammation in mice model of atopic dermatitis. β-glucans, a glucose polymer with glycosidic bonds and a component of S. cerevisiae cell wall, have been demonstrated to enhance tissue granulation and reepithelialization and a purported role in wound healingby activation of Dectin-1 receptor.
A repertoire of bioactives of intestinal tract origin, generated by a distinguished collection of microbial species, labor to safeguard cutaneous homeostasis and epithelial barrier function, namely, vitamin K, B12, butyrate and propionate. It does not spring a surprise to cognize in number of gastrointestinal disorders, cutaneous manifestations are prominent such as celiac disease with its dermatitis herpetiform or food allergies found in atopic dermatitis and psoriasis. Equally striking, acne patients show less diversity of gut microbiome with decreased level of Firmicutes and more abundance of bacteroids, a finding commensurate with amelioration of the lesions with use of probiotics. IGF-1, a major regulator of sebaceous lipogenesis with a well-known contribution to pathology of acne vulgaris, is consumed by probiotic bacteria, in particular lactobacilus, resulting in less IGF-1 in fermented milk as opposed to skim milk.
Sapienic acid, 16: 1n-6, is the most abundant fatty acid of the human sebum has been found to curb gram positive bacterial growth while in combination with ethanol, in low concentration, appeared far more efficacious than mupirocin. One mechanism by which sebum enhances cutaneous immunity against microorganisms is by upregulation of antimicoribal peptides as it was suggested by activation of beta defensin-2 by sebaceous free fatty acids, oleic acid, palmitic acid and lauric acid.
The stratum corneum despite being desiccated is a viable organ with both the bricks, corneocytes, and the cement, the matrix, possessing bioactivity with a fine sensitivity to precept subtle nuances in its equilibrium. Differentiating keratinocytes release, by exocytosis of lamellar bodies, two groups of peptides, β-defensins and cathelicidines, whether constitutionally such as β-defensin-1, cathelicidine LL-37, or upon exposure to microbial agents, inflammation or pollution, o3 and production of reactive oxygen species, β-defensin-2 and S100A12. These peptides can disrupt the cellular membrane of the pathogens or penetrate the cells to derange the intracellular functions to protect the skin.
Atopic dermatitis patients, who show increased S. aureus skin infections, have been shown having suppressed activity or less concentration of beta-defensins and cathelicidines as compared to patients with psoriasis who express higher level of antimicrobial peptides and lower susceptibility to cutaneous infections. Interleukin-4 and interleukin-13 account for enhanced susceptibility to cutaneous pathogens, bacterial, fungal and viruses in atopic dermatitis in which depleted sphingosines were reported.
1. Contassot E, French LE. New Insights into Acne Pathogenesis: Propionibacterium Acnes activates the inflammasome. J Invest Dermatol 2014 Feb;134(2):310-313
2. Mclaughlin J, Watterson S, Layton AM, et al. Propionibacterium acnes and acne vulgaris: New insights from the integration of population genetic, multi-Omic, biochemical and host-microbe studies.Microorganisms 2019 May; 7(5): 128
3. Agak GW, Kao S, Ouyang K, et al. Phenotype and antimicrobial activity of Th17 cells induced by Propionibacterium acnes strains associated with healthy and acne skin. J Invest Dermatol 2018 Feb; 138(2): 316–324
4. Lomholt HB, Kilian M. Population Genetic Analysis of Propionibacterium acnes Identifies a Subpopulation and Epidemic Clones Associated with Acne. PLoS One. 2010; 5(8): e12277
5. Dagnelie MA, Corvec S, Saint-Jean M, et al. Cutibacterium acnes phylotypes diversity loss: a trigger for skin inflammatory process. J Eur Acad Dermatol Venereol 2019 Dec;33(12):2340-2348
6. Desbois AP, Lawlor KC. Antibacterial Activity of Long-Chain Polyunsaturated Fatty Acids against acne bacteria. Mar Drugs. 2013 Nov; 11(11): 4544–4557
7. Sommer P, Bormann C, Gotz F. Genetic and Biochemical Characterization of a New ExtracellularLipase fromStreptomyces cinnamomeus. Appl Environ Microbiol 1997 Sep;63(9):3553-60
8. Thomsen MB, Lomholt HB, Scavenious C, et al. Proteome Analysis of Human Sebaceous Follicle Infundibula Extracted from Healthy and Acne-Affected Skin. PLoS One. 2014; 9(9): e107908
9. Nakatsuji T, Kao MC, Zhang L, et al. Sebum Free Fatty Acids Enhance the Innate Immune Defense of Human Sebocytes by Upregulating β-Defensin-2 Expression. J Invest Dermatol. 2010 Apr; 130(4): 985–994
10. Tomida S, Nguyen L, Chiu BH, et al. Pan-Genome and Comparative Genome Analyses of Propionibacterium acnes Reveal Its Genomic Diversity in the Healthy and Diseased Human Skin Microbiome. mBio. 2013 May-Jun; 4(3): e00003-13
11. Kwon HH, Yoon JY, Park SY, et al. Analysis of distribution patterns of Propionibacterium acnes phylotypes and Peptostreptococcus species from acne lesions. Br J Dermatol. 2013 Nov;169(5):1152-5
12. Yu Y, Champer J, Agak JW, et al. Different Propionibacterium acnes Phylotypes Induce Distinct Immune Responses and Express Unique Surface and Secreted Proteomes. J Invest Dermatol. 2016 Nov;136(11):2221-2228
13. Kim HJ, Lee BJ, Kwon AR. The grease trap: uncovering the mechanism of the hydrophobic lid in Cutibacterium acnes lipase. J Lipid Res. 2020 May; 61(5): 722–733
14. Burkhart CG, Burkhart CN. Expanding the microcomedone theory and acne therapeutics: acne bacteria biofilm produces biological glue that holds corneocytes together to form plug. J Am Acad Dermatol. 2007 Oct;57(4):722-4
15. Jarrousse V, Castex-Rizzi N, Khammari A, et al. Modulation of integrins and filaggrin expression by Propionibacterium acnes extracts on keratinocytes. Arch Dermatol Res. 2007 Nov;299(9):441-7
16. Lai Y, Cogen AL, Radek KA, et al. Activation of TLR2 by a Small Molecule Produced by Staphylococcus epidermidis Increases Antimicrobial Defense against Bacterial Skin Infections. J Invest Dermatol. 2010 Sep; 130(9): 2211–2221
17. Yuki T, Yoshida H, Akazawa Y, et al. Activation of TLR2 Enhances Tight Junction Barrier in Epidermal Keratinocytes. J Immunol September 15, 2011, 187 (6) 3230-3237
18. Tax G, Urban E, Palotas Z, et al. Propionic Acid Produced by Propionibacterium acnes Strains Contributes to Their Pathogenicity. Acta Derm Venereol 2015; 96(1)
19. Shu M, Wang Y, Yu J, et al. Fermentation of Propionibacterium acnes, a Commensal Bacterium in the Human Skin Microbiome, as Skin Probiotics against Methicillin-Resistant Staphylococcus aureus. PLoS One. 2013; 8(2): e55380
20. Sanford JA, O’Neil AM, Zouboulis CC, et al. Short-chain fatty acids from acne bacteria activate both a canonical and epigenetic inflammatory response in human sebocytes. J Immunol. 2019 Mar 15; 202(6): 1767–1776
21. Jalian HR, Liu PT, Kanchanapoomi M, et al. All-Trans Retinoic Acid Shifts Propionibacterium acnes-Induced Matrix Degradation Expression Profile toward Matrix Preservation in Human Monocytes. J Invest Dermatol 2008 Dec; 128(12): 2777-2782
22. Zeng R, Xu H, Liu Y, et al. miR-146a Inhibits Biofilm-Derived Cutibacterium acnes–Induced Inflammatory Reactions in Human Keratinocytes. J Invest Dermatol 2019 Dec 139(12): 2488-2496
23. Qin M, Pirouz A, Kim MH, et al. Acne bacteria induce IL-1β secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014 Feb; 134(2): 381–388
24. Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007 Jan;14(1):10-22
25. Guy R, Green MR, Kealey T. Modeling Acne in Vitro. J Invest Dermatol. 1996 Jan; 106(1):176-182
26. Mastrofrancesco A, Kokot A, Eberle A, et al.KdPT, a Tripeptide Derivative of α-Melanocyte–Stimulating Hormone, Suppresses IL-1β–Mediated Cytokine Expression and Signaling in Human Sebocytes. J Immunol. 2010 Aug: 185(3) 1903-1911
27. Skabytska Y , Biedermann T. Staphylococcus epidermidis Sets Things Right Again. J Invest Dermatol. 2016 March: 136(3): 559-560
28. Wang Y, Kuo S, Shu M, et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of acne bacteria: Implications of probiotics in acne vulgaris. Appl Microbiol Biotechnol. 2014 Jan; 98(1): 411–424
29. Shahzeb H, Christian P, Junaid B, et al. Saccharomyces cerevisiae as a skin physiology, pathology, and treatment model. Dermatol Online J. 2020 Nov 15;26(11):13030
30. Epinga H, Bing Thio H, Schreurs MWH, et al. Depletion of Saccharomyces cerevisiae in psoriasis patients, restored by Dimethylfumarate therapy (DMF). PLoS One. 2017; 12(5): e0176955
31. Nenoff P, Muller B, Sander U, et al. IgG and IgE immune response against the surface glycoprotein gp200 of Saccharomyces cerevisiae in patients with atopic dermatitis. Mycopathologia. 2001;152(1):15-21
32. Yeh CY, Jung CJ, Huang CN, et al. A legume product fermented by Saccharomyces cerevisiae modulates cutaneous atopic dermatitis-like inflammation in mice. BMC Complement Altern Med. 2014; 14: 194
33. Majtan J, Jesenak M. β-Glucans: Multi-Functional Modulator of Wound Healing. Molecules. 2018 Apr; 23(4): 806
34. Scott KP, Gratz SW, Sheridan PO, et al. The influence of diet on the gut microbiota. Pharmacol Res. 2013 Mar;69(1):52-60
35. Sikora M, Stec A, Chrabaszcz M, et al. Gut Microbiome in Psoriasis: An Updated Review. Pathogens. 2020 Jun; 9(6): 463
36. Fabbrocini G, Bertona M, Picazo O, et al. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef Microbes. 2016 Nov 30;7(5):625-630
37. Bowe WP, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis – back to the future? Gut Pathog. 2011; 3: 1
38. Baldwin H, Tan J. Effects of Diet on Acne and Its Response to Treatment. Am J Clin Dermatol. 2021; 22(1): 55–65
39. Kang SH, Kim JU, Kim JY, et al. The Effects of Dairy Processes and Storage on Insulin-Like Growth Factor-I (IGF-I) Content in Milk and in Model IGF-I–Fortified Dairy Products. J Dairy Sc. 2006 Feb 89(2): 402-409
40. Nakatsuju T, Kao MC, Zhang L, et al. Sebum Free Fatty Acids Enhance the Innate Immune Defense of Human Sebocytes by Upregulating β-Defensin-2 Expression. J Invest Dermatol. 2010 Apr; 130(4): 985–994
41. Nakatsuju T, Kao MC, Fang JY, et al. Antimicrobial Property of Lauric Acid Against Propionibacterium acnes: Its Therapeutic Potential for Inflammatory Acne Vulgaris. J Invest Dermatol. 2009 Oct; 129(10): 2480–2488
42. Drake DR, Brogden KA, Dawson DV, et al. Thematic Review Series: Skin Lipids. Antimicrobial lipids at the skin surface. Thematic Review. 2008 Jan;49(1):4-11
43. Fischer CL, Walters KS, Drake DR, et al. Sphingoid bases are taken up by Escherichia coli and Staphylococcus aureus and induce ultrastructural damage. Skin Pharmacol Physiol. 2013; 26(1): 36–44
44. Bibel DJ, Aly R, Shinefield HR. Antimicrobial activity of sphingosines. J Invest Dermatol. 1992 Mar;98(3):269-73
45. Stolzenberg ED, Anderson GM, Akermann MR, et al. Epithelial antibiotic induced in states of disease. Proc Natl Acad Sci U S A. 1997 Aug 5; 94(16): 8686–8690
46. Woodby B, Pambianchi E, Ferrara F, et al. Cutaneous antimicrobial peptides: New “actors” in pollution related inflammatory conditions. Redox Biol. 2021 May; 41: 101952
47. Gallo RL, Huttner KM. Antimicrobial Peptides: An Emerging Concept in Cutaneous Biology. J Invest Dermatol. 1998 Nov; 111(5):739-743
48. Ong PY, Ohtake T, Brandt C, et al. Endogenous Antimicrobial Peptides and Skin Infections in Atopic Dermatitis. N Engl J Med 2002 Oct; 347:1151-1160
49. Christophers E, Henseler T. Contrasting disease patterns in psoriasis and atopic dermatitis. Arch Dermatol Res
. 1987;279 Suppl:S48-51
50. Ma JY, Shao S, Wang G. Antimicrobial peptides: bridging innate and adaptive immunity in the pathogenesis of psoriasis. Chin Med J (Engl). 2020 Dec 20; 133(24): 2966–2975
51. Arikawa J, Ishibashi M, Kawashima M, et al. Decreased Levels of Sphingosine, a Natural Antimicrobial Agent, may be Associated with Vulnerability of the Stratum Corneum from Patients with Atopic Dermatitis to Colonization by Staphylococcus aureus. J Invest Dermatol. 2002 Aug; 119(2):433-439